Prof. K. VijayRaghavan - Development of neural circuits and muscles
| People | |
|
Ajeet Pratap Singh & Rudra Nayan Das
Gururaj |
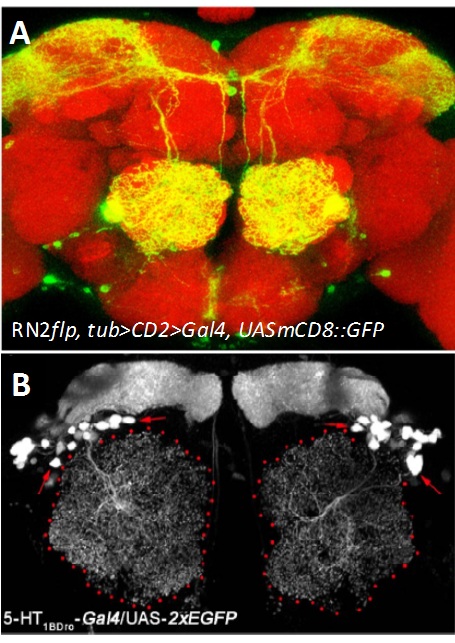
Developmental mechanisms and modulatory functions of a central serotonergic neuron in the Drosophila antennal lobe. Serotonin, 5-hydroxytryptamine (5-HT) is an evolutionarily ancient monoamine and plays diverse roles in the brain. Serotonergic dysfunction is implicated in various neuropsychological disorders such as anxiety and depression as well as in neurodegenerative disorders. In Drosophila, multiple studies provide evidences for involvement of serotonin in innate behaviours like sleep, circadian rhythm, courtship, aggression and learning. Driven by the keen desire to understand the basic mechanism by which serotonin regulates these aspects we focused our study on an identified pair of serotonergic interneuron innervating the antennal lobe, called the CSDn (contralaterally-projecting, serotonin-immunoreactive deutocereberal neuron, Figure- Right panel). Using mosaic method that unilaterally labels the CSDn, we observed a glomerular-specific distribution of its axonal terminals which was put in place during development, by the differential levels Eph-Ephrin signaling. Using variety of genetic tools and behavioral paradigms, we demonstrated that the CSDn modulates olfactory sensitivity in an odor specific manner and the differential distribution of axonal terminals was necessary for this modulation. Further, our work suggests the involvement of one of the receptors of serotonin, 5-HT 1B (Figure- Left panel) in this serotonergic modulation of olfactory information.
Figure: (A) The CSDn (green) in an adult brain (Red). (B) Expression pattern of 5-HT1BDro-Gal4 in a subset of interneurons and mushroom body neurons. Collaborator: Mathias Landgraf , Department of Zoology, University of Cambridge, UK
|
|
Sonia Sen
Ramveer Choudhary
|
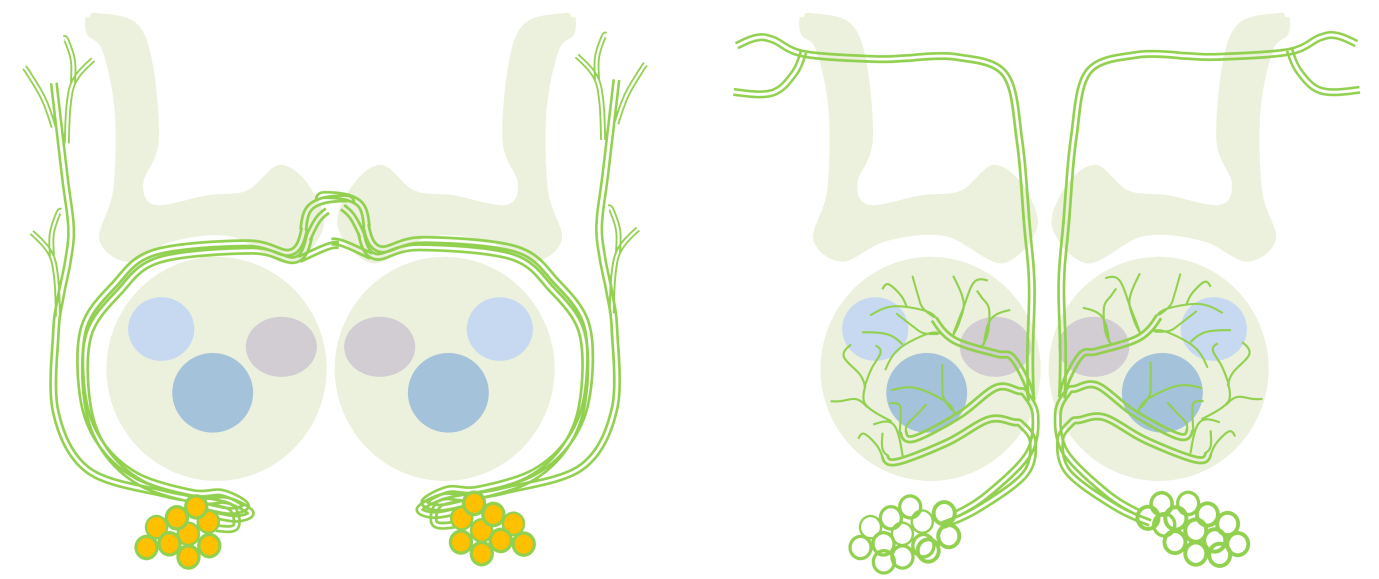
Conserved peripheral and central roles of transcription factors, empty spiracles and orthodenticle, in Drosophila olfactory system development The Drosophila brain develops from neural stem cell like precursros called neuroblasts, which form in a highly stereotyped manner in during embryogenesis. The stereotypy in the formation and specification of embryonic neuroblasts is in part controlled by key developmental patterning genes that initially define the body axes during early embryogenesis. Many of these genes continue to be expressed in neuroblasts later in embryonic and postembryonic brain development, and have functional roles there. We investigate some of these later, complex roles of early patterning genes. We have focussed on two cephalic gap genes orthodenticle (otd) and empty spiracles (ems) and are studying their role in postembryonic development of the Drosophila central nervous system and peripheral nervous system, specifically in the olfactory system. We find that these genes continue to be required reiteratively in the peripheral and central neurons of the olfactory system. Our work suggests that proper development of the circuit depends not only on genes that are expressed in olfactory lineages, which promote appropriate circuitry, but also on genes expressed elsewhere, which prevent inappropriate circuitry.
In this schematic, a neuroblast lineage that normally does not innervate the antennal lobe (left) expresses the transcription factor Otd (orange). Loss of otd function from this lineage results in the transformation of its neuronsto antennal lobe projection neurons (right). Collaborators: Prof. Heinrich Reichert, Biozentrum, University of Basel, Basel, Switzerland Rahul Siddharthan, The Institute of Mathematical Sciences, Chennai, India |
| Indu Nair |
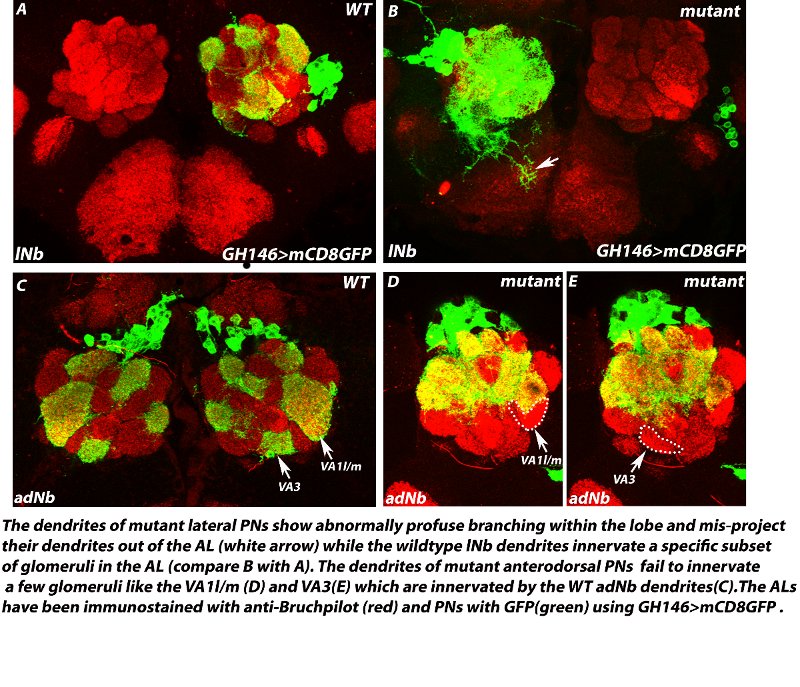
A zinc-finger transcription factor regulates the guidance of axons and dendrites of the olfactory neurons in Drosophila melanogaster Precise guidance of growing axons and dendrites to their proper targets is indispensable for the proper development of any neural circuit which is a prerequisite for normal behaviour in animals. In the fruitfly, the olfactory receptor neurons (ORNs), typically express a single OR gene and all the ORNs expressing the same OR gene extend their axons precisely to one specific glomerulus out of about 50 glomeruli within the antennal lobe (AL), the first olfactory relay in the brain. The second order interneurons viz., the projection neurons and local interneurons also send their dendrites to specific target glomeruli to receive inputs from their respective ORN partners. Several axon guidance and cell surface molecules like have been shown to be required in this guidance process. However, there is very little understanding of the upstream transcription factors that control axon/dendrite guidance. We are investigating the role of a zinc-finger transcription factor in the guidance of axons of ORNs and dendrites of PNs and LNs during development of the Drosophila olfactory circuit. Loss-of-function of this factor causes specific mistargeting of a subset of ORN axons to one dorsolateral glomerulus in the antennal lobes. The dendrite projections of the PNs belonging to the anterodorsal lineage also show loss of innervation to specific glomeruli and the dendrites of lateral PNs and LNs misproject out of the ALs. Our study has identified a new player- a zinc finger transcription factor that seems to be part of the transcription factor code that determines the targeting of individual neurons in the olfactory system.
Collaborators: Prof. Heinrich Reichert, Biozentrum, University of Basel, Basel, Switzerland |
|
Aman Aggarwal |
Development of interlimb coordination in adult Drosophila Locomotion is a very important behaviour of animals. To move around efficiently the limbs involved should move in a coordinated fashion. Even though many animals learn to walk after birth, coordination between limbs is seen as soon as they are born. How this interlimb coordination develop without any real world experience is an open question in the field. Using Drosophila as a model system, we are trying to figure out how this happens. Adult Drosophila walks in a highly coordinated fashion as soon as it is born. We have established a new assay where we can track the leg movements in pupa before the adult is born. Using this assay we are trying to address how interlimb coordination develops in animals. |
|
Swetha B.M
Pushkar Pranjape |
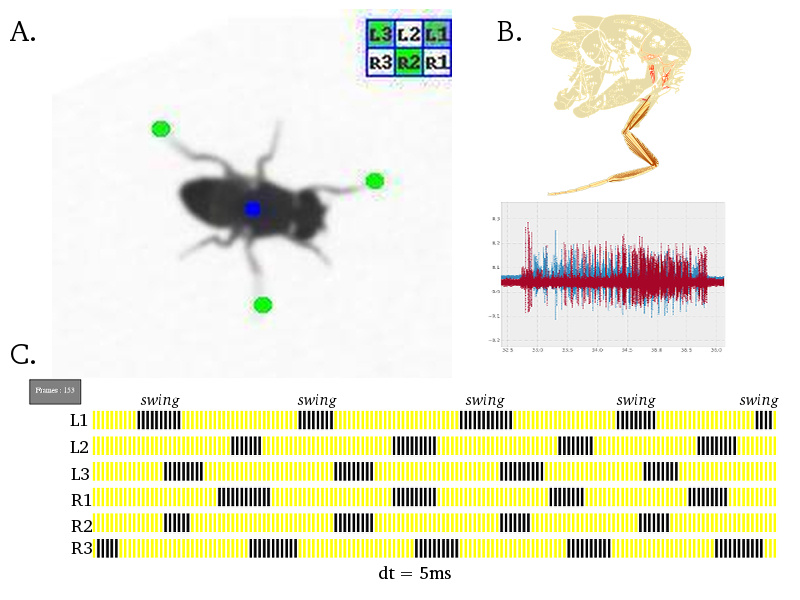
Neural Circuit Mechanisms Underlying Co-ordinated Motor Neuron Activity of Freely Walking Drosophila Walking is a complex motor behavior that demands sequential and periodic contraction of muscles. We have a substantial understanding of the organization and developmental assembly of motor neuron dendritic arbors and axonal projections to the leg muscles in Drosophila (Brierley et al., 2009, 2011). But the neural circuit connectivity of pre-motor inter neuronal elements is poorly understood. The role of specific gene products at these pre-motor synapses has been difficult to pin-down in any organism. We have developed high throughput behavioral assays and single fly electro-physiological preparations in the laboratory to quantify walking behaviour at a single leg resolution. A battery of powerful genetic reagents, some of which are developed in-house, has enabled sophisticated spatio-temporal interrogation of the molecular and neural circuit function. We find that perturbing the pre-motor inhibitory inputs results in drastic walking defects and have refined this analysis to exclude potential developmental and non-specific effects. Efforts are under-way to explain the precise role of inhibitory neurotransmitter receptors in a defined cellular and behavioral context of walking.
Figure: A. Freely walking adult Drosophila is captured on a 200 frames/second camera. Using a custom algorithm invented in the laboratory, co-ordinates of all six leg tips are identified automatically; green dot indicates leg tip, blue dot indicates fly centroid and a leg activity matrix summarises the inter-leg swing co-ordination state. B. Drosophila leg musculature (modified from Demerec, 1950) and intra-cellular electrical recordings for a pair of muscles. C. A "gait diagram" is generated automatically from the data extracted in A. and summarises a single walking bout of a fly; blue spikes indicate swing phase and yellow spikes represent stance/stationary phase; each spike is 5ms in duration. |
|
Syed Durafshan Sakeena |
Mechanism underlying the development of functional motor neuron connectivity in the Drosophila walking circuit
Complex behaviors such as escaping predators, finding food, or attracting a potential mate require coordinated movement. An animal gathers sensory cues, integrates this information with the final outcome an appropriate motor behavior. We are studying the mechanism underlying the development of one form of motor output, walking circuit in adult Drosophila.
Figure : Organization of a late born motoneuron (A) Projection pattern of dendrites of late born motor neuron in the lateral region of ventral nerve cord (VNC) and (B) Axonal projection of a late born neuron innervating distil muscle of Tibia. Collaborators: Prof. Heinrich Reichert, Biozentrum, University of Basel, Basel, Switzerland |
|
Ali Asgar Bohra |
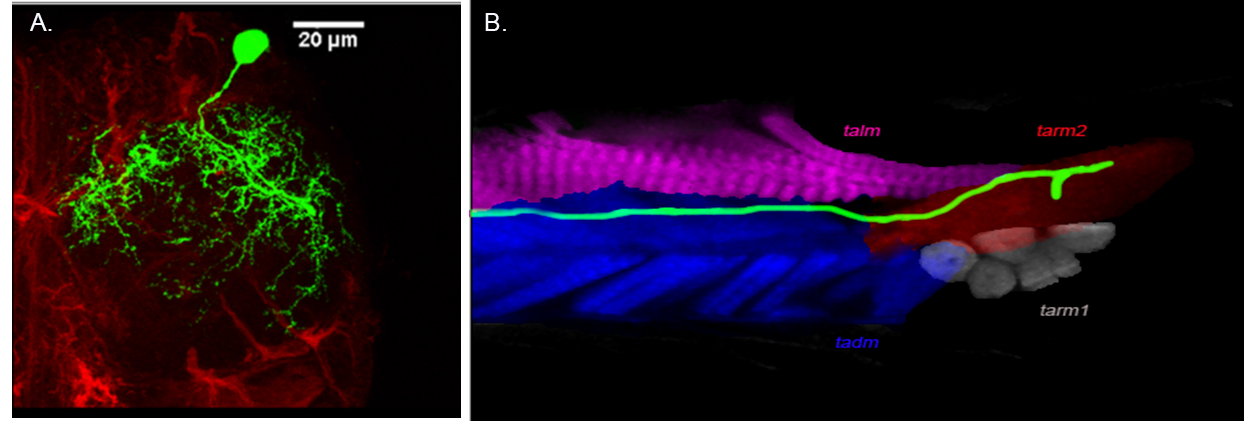
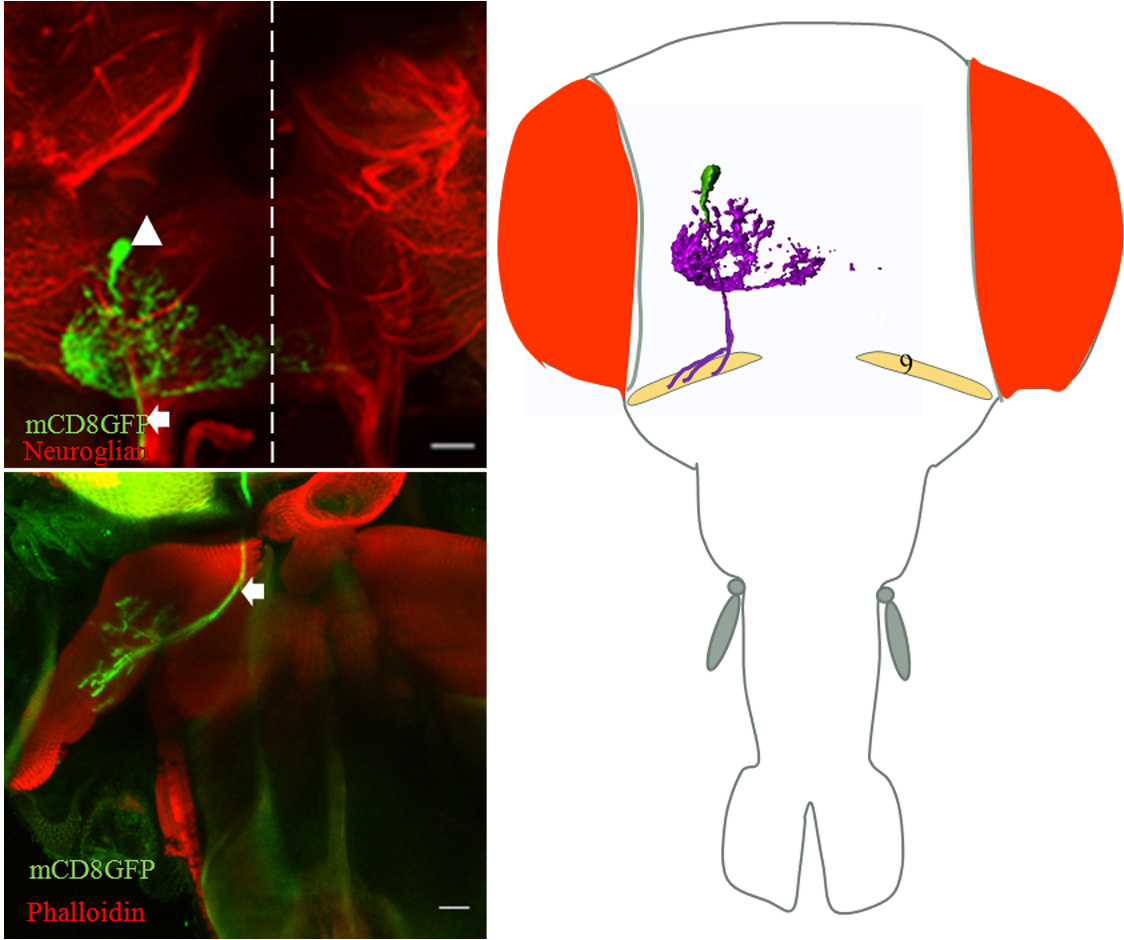
Developmental analysis of gustatory motor neurons: Organization, birth order and targeting. Adult Drosophila feed by the contraction of multifibrous muscles present in the proboscis. These muscles are controlled by the axons of the proboscis motoneurons, whose correct wiring during development is critical for normal muscle function. Functional motor connectivity also requires the dendrites of the motor neurons to be correctly placed and connected in the neuropil in the brain. The origin, organization and innervation of the motor neurons to the proboscis muscles are poorly characterized. We are analyzing the development and origin of the motor neurons that innervate the proboscis muscles. We are also studying the development of single motoneuron throughout the development. This study will help us in understanding the formation of the neural circuit in general and gustatory motor output circuit in particular.
Figure: Single cell MARCM clone innervates protractor of fulcrum (muscle 9) (a) cell body of motor neurons in SOG (arrowhead) and axon tract (arrow) out from brain. (b) Innervation of protractor of fulcrum in proboscis by the motor neuron axon (arrow). (C) Schematic showing position of motor neuron in SOG and innervation in respective proboscis muscle. Collaborator: Prof. Heinrich Reichert, Biozentrum, University of Basel, Basel, Switzerland |
|
Venkateswara Reddy Onteddu |
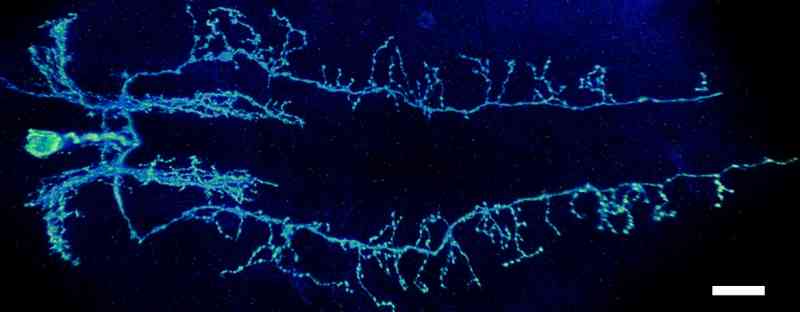
Identification of the organization of Drosophila glutamatergic neurons. My study is focus on identifying the organization of glutamatergic neurons in the insect Drosophila. The interaction of sensory and interneurons of the nervous system is required to instruct motor neurons for muscle contraction. The information contained and processed in these neurons is mainly determined by their organization in nerve cord. Identification of the spatial organization of interacting neurons will enable predicting the outcome of their interactions and subsequent movement related behaviours.
Figure: An image of a glutamatergic interneuron having neurites along the length of nerve cord. Scale bar 20µm |