Prof. K. VijayRaghavan - Signalling mechanisms of tissue morphogenesis
|
|
Muscle Development - Attachment
Proper organization of muscle-tendon pattern requires communication during development between these two cell types. Factors that regulate tendon cell development, specificity, and the mechanisms through which they communicate with muscle cells are not fully understood. We took two approaches to identify new mechanisms that regulate this process. First, we carried out a genome-wide RNAi screen, using hairpin-RNAi constructs expressed in developing tendon cells. This screen picked 24 candidates in which there are transcription factors, enzymes, intracellular transport molecules, cell surface molecules and molecules of unknown function. Further analysis suggests important role of these candidates during tendon cell differentiation and junction formation. Second, we analyzed the role of known signaling pathways during junction formation. Compromising RTK as well as wingless signaling pathways in tendon cells leads to defects in tendon cell maturation and junction formation. Muscle-tendon junction formation and stabilization in Drosophila requires secretion of structural proteins. Extracellular matrix (ECM) molecules secreted at the junction by tendon cells and cell-adhesion molecules present on both the cell types play a crucial role in formation, maturation and maintenance of the junction. The tango1 gene product localizes to the endoplasmic reticulum exit site and is involved in secretion. We find that tendon cell-specific knockdown of tango1 leads to loss of the muscle tendon junction during pupal development. We also show that accumulation of thrombospondin (an extracellular matrix molecule) at the junction is affected in tendon cell specific tango1 knockdown. Collagen IV is another ECM molecule which is a major constituent of the basement membrane. Collagen IV has been shown to be secreted by hemocytes and fat bodies. We find that loss of tango1 in these cells inhibits secretion of Collagen IV.
Figure 1- Tendon specific knockdown of CG33303 leads to disorganized tendon cells and affects muscle development. Muscles are marked by MHC-GFP (green), tendon cells are marked by sr-Gal4,UAS-mRFP (red). |
|
|
Investigating myoblast stemness in the context of notch and epidermal signaling.
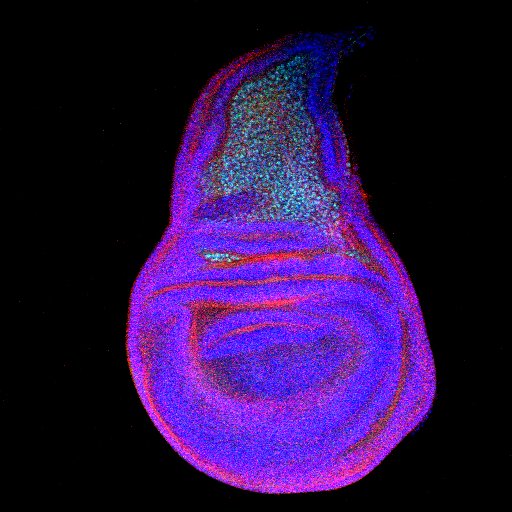
How organ size is regulated is something underlies most of the developmental processes and is a mystery and muscle is a complex tissue. Muscle size varies greatly depending upon the function involved in and essentially governed by the number of units, myoblasts used for its formation. The numbers of myoblasts for each muscle are roughly defined and are formed during early developmental stages. How few precursors, AMPs (Adult muscle precursors) form a large number of myoblasts is something we wanted to know. This process is very similar in many organisms and the large flight muscles of Drosophila used extensively during maneuvering provide excellent opportunity to address this question. Using various genetic experiments complemented by lineage tracing we could identify previously unidentified stem cell population. Along with stem cells, experiments performed unraveled signaling niche, a structure that provides all essential growth signal in the wing imaginal disc.
Figure: The third instar wing disc showing stem cells (green) in the presumptive notum marked by D-Cad2 (red). |
|
Sphingolipid signaling in muscle development My work is a collaboration with Julie Saba of the Children’s Hospital Oakland Research Institute in Oakland, CA, USA, to study the role of sphingolipid metabolites in muscle and motor circuit development. Over the past two decades, sphingolipids have come to be recognized as an important class of signaling lipids that regulate cell proliferation, survival, migration and differentiation. Among these lipids, sphingosine-1-phosphate (S1P) has been investigated for its role in several diseases. The levels of S1P in vivo are regulated by the enzyme sphingosine-1-phosphate lyase, which in Drosophila is encoded by the gene sply. Sply irreversibly degrades S1P and thus regulates flux through the sphingolipid metabolic pathway. Reduced Sply activity in Drosophila leads to missing Indirect Flight Muscles in the adult. We have established that this is due, at least partly, to defects in early events of muscle development. This phenotype closely resembles defects seen upon the disruption of several other factors, such as Wg and Notch signaling, and motor neuronal influences. Ongoing experiments are focused on determining the etiology of the sply loss-of-function phenotype, and to this end, we are investigating putative interactions between intercellular signaling mechanisms and components of the sphingolipid metabolism pathway. These studies will contribute to a better understanding of sphingolipid mediated signaling in developmental contexts, and expand on the use of Drosophila, a genetically tractable model organism, in the investigation of sphingolipids.
Collaborators: Julia Saba |