Prof. M. K. Mathew - Research
Endocytic Mechanisms in Salt Tolerance (Anirban Baral, in collaboration with Prof. S Mayor, NCBS):
Endocytosis, the internalization of plasma membrane along with embedded proteins and extracellular fluid, is a ubiquitous cellular process in metazoans. Multiple pathways of endocytosis have been identified in animal systems which vary in terms of the molecular constituents involved and also by the type of cargo that is internalized. Most studies have utilized isolated cells in culture apart from some recent studies in developing embryos of C elegans and Drosophila. The latter studies raise the possibility that endocytic processes may be differentially regulated across different cell lineages. Endocytic mechanisms may be expected to vary across cell types in an intact, functional tissue and, moreover, be subject to differential regulation in response to varying physiological conditions. The Arabidopsis root is a well stratified organ composed of distinct cell layers which are clearly demarcated in terms of position, shape, developmental origin and gene expression profiles – and is amenable to imaging in its entirety, thus allowing the study of endocytosis in an intact functioning tissue. Exploiting the optical transparency and physical accessibility of young Arabidopsis roots we have explored the full panoply of endocytic mechanisms in the different cell layers. We have probed uptake mechanisms utilizing a range of probes and varied physiological conditions. We find that at least three distinct mechanisms of endocytosis operate in the root. A relatively well characterized clathrin-dynamin mediated mechanism operates across all cell layers and serves to take up transmembrane proteins. In addition, a clathrin-independent pathway operates constitutively in the epidermis – the outermost layer of the root. This pathway takes up lipid but excludes transmembrane proteins. Finally, salinity stress induces a clathrin-independent pathway in all layers of the root that is catholic in its choice of cargo, and employs molecular components that are not shared with the constitutive clathrin-independent pathway. Concomitant with the induction of this pathway, we observe the expansion of small acidic compartments into larger vacuole-like structures in inner cell layers. It may be noted that large vacuoles are a feature of the epidermis, but not seen in internal layers. Thus saline stress reprogrammes endocytic pathways and remodels a vital compartment involved in intracellular trafficking.
Mutant plants deficient in the third pathway fail to make large vacuoles in internal cell layers. They are also severely salt sensitive. We speculate that there could be a correlation between construction of mature vacuoles and the operation of the clathrin-independent endocytic pathways. In unstressed plants, the only cell layer with a well developed vacuole is the epidermis, which also has an active clathrin-independent uptake system. Under saline stress, the induction of a clathrin-independent endocytic process throughout the root correlates with the development of a vacuolar system in the inner layers. We therefore suggest that the salt-induced pathway of endocytosis contributes to the formation of large vacuoles in internal cell layers and is critical to the mounting of a successful defence against salinity stress.
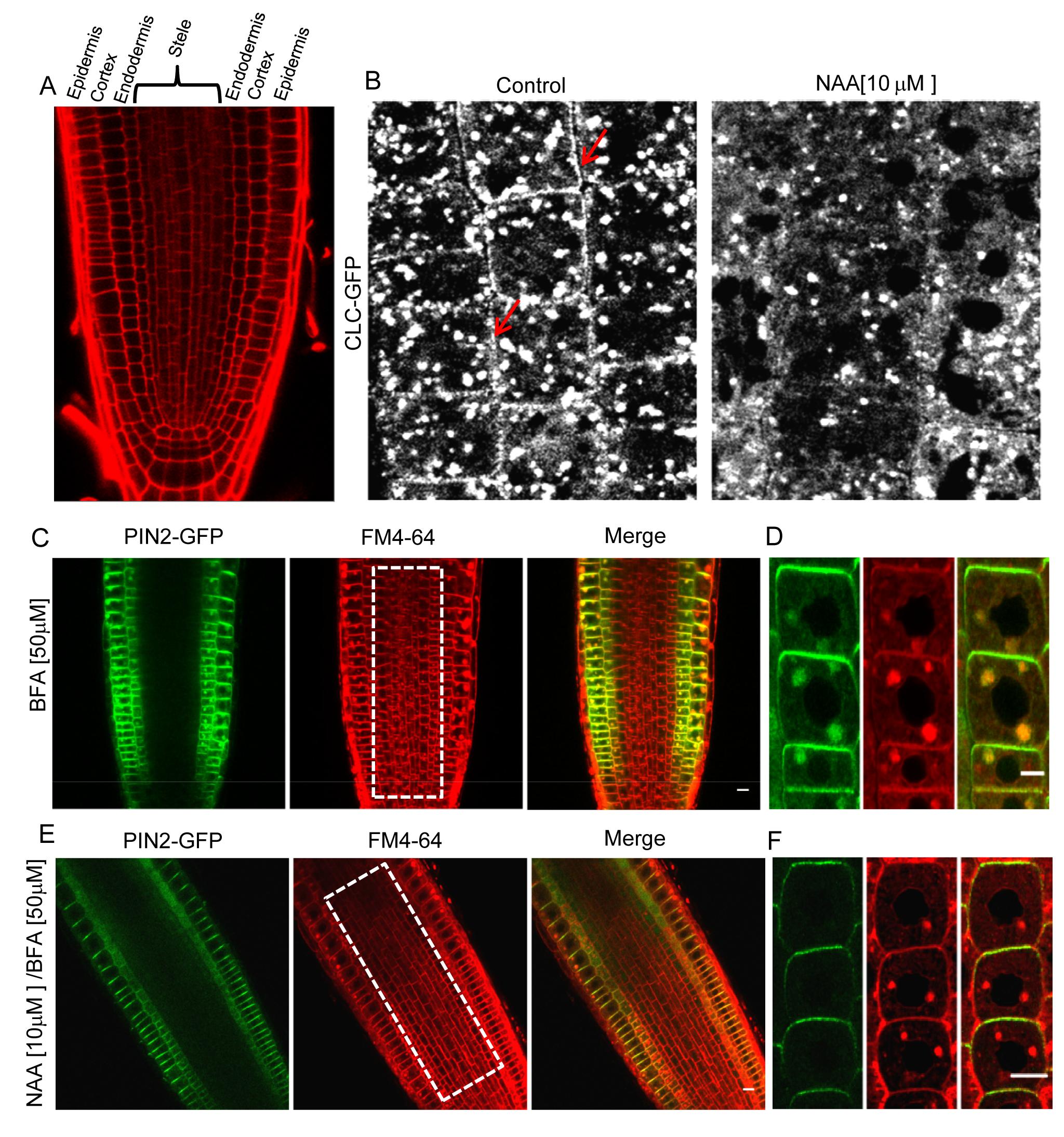
Fig 1A: Different layers of Arabidopsis root visualized by propidium iodide staining. The layers of the root from outside to inside are epidermis, cortex, endodermis and stele. Treatment with NAA reduces localization of clathrin localization to plasma membrane (Fig 1B, right) seen in control plants (Fig 1b, left, red arrows). Uptake and Brefeldin-A (BFA) induced clumping of transmembrane protein PIN2-GFP and lipid probe FM4-64 (Fig 1C and 1D). Pre-treatment with NAA prevents endocytosis and BFA induced clumping of PIN2-GFP but FM4-64 is still taken up in epidermal cells (Fig1E and 1F), indicating operation of a clathrin–independent endocytosis in those cells. Note that, Stele cells in BFA treated roots have clumps containing FM4-64 while those pre-treated with NAA do not. (1B and 1D, boxed area in middle) suggesting clathrin-dependent endocytosis is predominant in those internal cell layers. NB: BFA induced clumping of endocytosed cargo is a standard assay to monitor endocytosis in plants (Paciorek et al., 2005).
Root Architecture and under Drought and Salinity (Rukaya Amin, in collaboration with Prof. HE Shashidhar, University of Agricultural Sciences, GKVK Bangalore):
We have studied the architecture of roots of rice plants subjected to drought and saline stress. Four cultivated Indica varieties were chosen – Pokkali, which grows in coastal regions and is very tolerant to salt; BI-33, a drought tolerant cultivar which was developed in UAS; IR-20, which is very sensitive to both salt and drought; and Jaya, which is intermediate in its tolerance characteristics. Roots of the four varieties differ greatly in length and number in a manner that could contribute to their tolerance characteristics. Earlier work in the lab had shown that the integrity of a waxy barrier around the endodermis is critical for restricting the entry of Na+ into the xylem stream and hence its uptake into shoots. The ability to restrict Na+ upate into shoots correlates with salt tolerance. The waxy barrier has breaks for passage cells which allow fluid access. We now find that xylem sap uptake into the shoot correlates with the density of passage cells. This correlation differs strikingly between control plants and those subjected to either drought or saline stress. Xylem sap uptake increases almost linearly with passage cell density in control plants. All four varieties dramatically reduced xylem sap uptake into shoots on saline stress. On the other hand, there is a dramatic increase in passage cell number on drought stress together with a large spread in xylem sap uptake among the different varieties in response to drought. BI-33 shows a more than three-fold increase in passage cell density, which contributes to its ability to maintain high fluid uptake even under drought. The composition of the xylem sap has also been analysed. We find that Pokkali adds a large quantity of osmolytes to its xylem sap thereby compensating for the osmotic imbalance with the saline medium.
Our data indicate that BI-33 has very high xylem sap uptake in control plants, and its ability to maintain high uptake even under drought stress contributes to its survival under drought stress. BI-33 has very long roots that extend almost vertically downwards in order to tap into water reserves deep within the soil. Pokkali is very succesful in adding osmolytes to the xylem sap under saline stress and also in restricting Na+ entry into the sap, thus maintaining reasonable xylem flow under saline conditions without subjecting the shoot to Na+ loading.
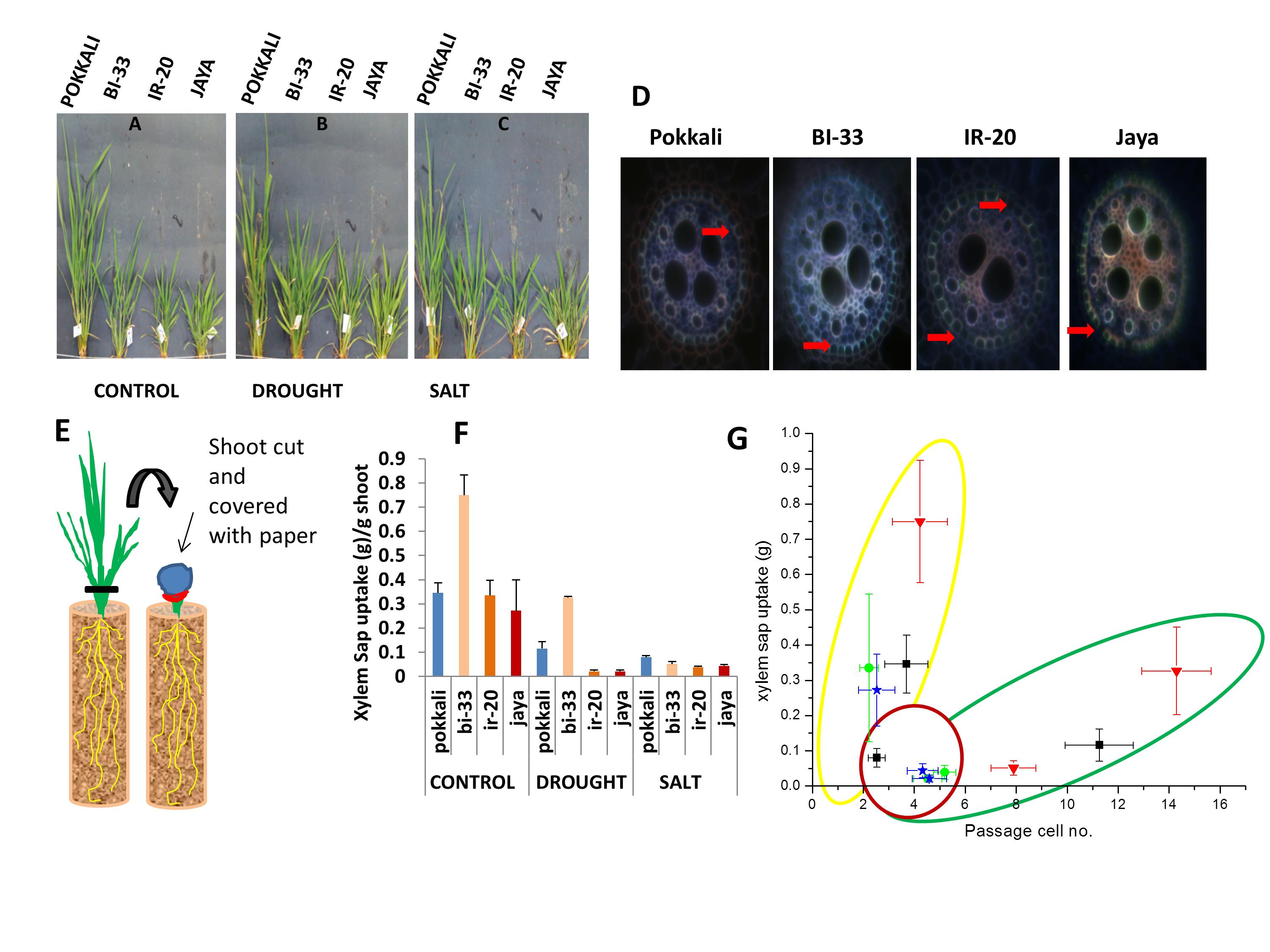
Figure 2: Shoot of different rice varieties at 45 days. Plants were subjected to A) control (well-watered), B) drought (no irrigation) and C) salinity (100mM) from the 38th day for one week. D) Passage cells. For passage cell scoring, root sections (200mm) taken from control, drought and salt stressed plants were stained with berberin hemisulphate and aniline blue. Sections were observed under fluorescent microscope using UV filter and number of cells with no tangential suberin or no suberin deposition at all, were counted. E) Schematic Representation of xylem sap uptake. After growing rice varieties in PVC pipes and exposing them to control (well-watered), drought (no irrigation) and salt (100mM) conditions from the 38th day, for about a week. After 45th day Shoot was cut 5cm above soil level and the stem covered first with blotting paper and then polyethylene wrapper, which was then tightly sealed with rubber band. After 12 hours, blotting paper was weighed to obtain the mass of xylem sap collected. F) Quantification of xylem sap uptake in control, drought and salt condition. G) Relation between passage cell number and xylem sap uptake in different varieties of rice (Pokkali, BI-33, IR-20 and Jaya). Data points cluster according to the treatment given and outlines have been drawn for clarity. Yellow – control plants, Red – plants subjected to salinity and Green – plants subjected to drought.
VDAC and Cell Death (Ashvini Dubey & Ashwini Godbole):
The voltage-dependent anion-selective channel (VDAC) is the most abundant protein in the mitochondrial outer membrane and forms the major conduit for metabolite transport across this membrane. We had earlier shown that VDAC plays a role in cell death in both plants and animals. Bcl2-family proteins that regulate mitochondrially-mediated cell death in animal cells have been shown to interact with VDAC, and this interaction has been implicated in some models of their regulation of cell death. Further, hexokinase has been suggested to modulate cell death by interacting with VDAC. We had shown earlier that heterologously expressed VDAC is initially present in the cytosol and subsequently moves to the mitochondrion. An analysis of the distribution of heterologously expressed VDAC in cells which also overexpress hexokinase or Bcl2-family proteins indicates that death correlates with the fraction of expressed VDAC that was localized in mitochondria as opposed to the cytosol. We therefore propose that one mechanism by which Bcl2-family proteins and hexokinase regulate cell death is by controlling the distribution of freshly synthesised VDAC between the cytosol – its site of synthesis – and the mitochondrion.
Biophysical Characterization of VDAC (Ashvini Dubey & Ashwini Godbole):
OsVDAC4 is a rice VDAC which we have previously shown to operate in an apoptotic context in lymphocytes. OsVDAC4 protein was purified after overexpression in E coli and reconstituted into artificial membrane systems of two topologies –planar bilayer membranes which allow for electrical measurements, and spherical liposomes which are suitable for monitoring solute fluxes. We monitored liposome swelling in response to influx of polyethylene glycols of defined sizes through VDAC to estimate a pore radius of around 1.3 nm.
Electrical measurements made on single channels inserted into planar bilayers revealed a unitary conductance of 4.53 nS with several subconductance states. The probability of channel opening was maximal at 0 mV and decreased on shifts to either positive or negative potentials, with more transitions seen at positive potentials than at negative potentials. Introduction of ATP into the chamber resulted in a decrease in measured conductance (due to the decreased mobility of ATP compared to Cl-) and an increase in noise. We also estimated the amount of ATP transported through a single channel to be around 107 ATP per second at -10 mV with an imposed gradient of 50 mM ATP.
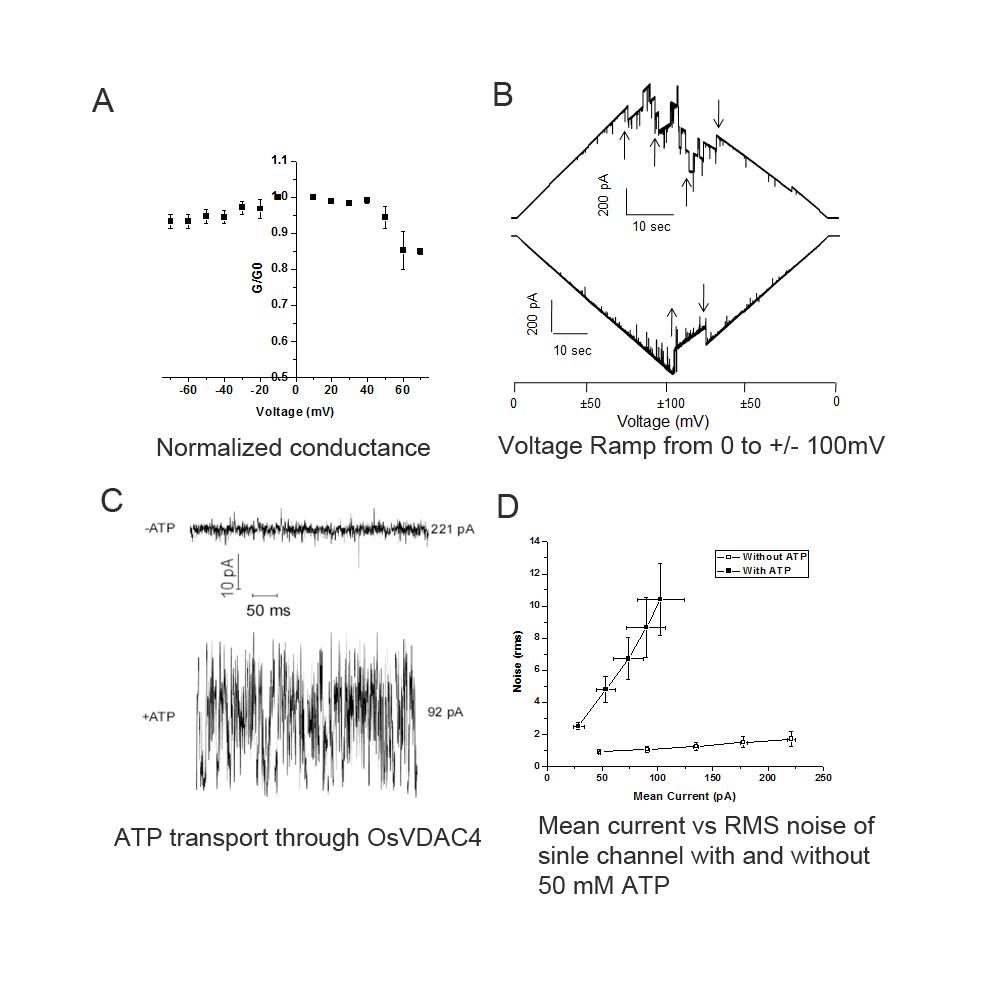
Fig 3. Electrophysiological characterization of OsVDAC4. A) Voltage dependence of OsVDAC4 conductance. Membrane conductance normalized to the conductance at +10 mV (G/G10) as a function of applied membrane potential (mV) (n = 5). B) Voltage Ramp. Membrane containing 3 OsVDAC4 channels was subjected to 2 voltage ramps. In the upper trace, voltage was varied from 0mV to +100 and back to 0mV and in the lower trace from 0mV to -100 and back to 0 mV. The voltage was ramped at 5mV/sec. Transitions between sub conductance states are indicated with arrows. C) ATP transport through OsVDAC4, A representative trace at 50 mV in the absence and presence of 50 mM ATP under symmetric buffer conditions. Note the large increase in noise accompanied by a decrease in current on introduction of ATP. D) RMS noise and mean conductance through single channels in the presence and absence of 50 mM ATP and potentials ranging from +10 to +50 mV. Each point represents mean current and noise (±SE) from four independent channel recordings. Empty square: channel in the absence of ATP, Filled square: channel in the presence of 50 mM ATP.



